ASK MY PHARMACIST | GOT QUESTIONS? Your pharmacist has answers. Click Here
PSI Ireland Exam for Indian Pharmacists
Indian pharmacists to Ireland guide for TCQR process explaining application process, why choose Ireland, eligibility and tips
Sahana
6/24/20255 min read


For Indian pharmacist aspiring to practise abroad, Ireland provides with great opportunities. If you are aiming to build a successful pharmacy career abroad, the Pharmaceutical Society of Ireland (PSI) is the regulatory body responsible for the registration of pharmacists in Ireland. This process falls under "Third Country Qualification Recognition (TCQR) pathway." For overseas-qualified pharmacists—including those from India—this a multi-step process where clearing the PSI’s qualification recognition process is an essential step towards working as a licensed pharmacist in Ireland.
Key aspects that you’ll here:
PSI Eligibility
Application steps
Why Ireland is the choice for Indian pharmacists
Differences in the old and new process.
Exams for PSI registration.
Tips for Indian pharmacists planning to register in Ireland.
Why Choose Ireland for Your Pharmacy Career?
There are many reasons for Indian pharmacists to get attracted towards a pharmacy career in Ireland and a few of them are listed for you to guide you through the decision of why choose Ireland:
Unlike India, Ireland offers competitive salaries and quality work-life balance
High standards of living when compared to India.
Even in the economic turndowns, the healthcare sector in Ireland is has been robust and resilient.
The shortage of skilled professionals like Pharmacists have a great demand in both urban and rural regions.
Opportunities in hospital, community, and clinical pharmacy
The registration process of Ireland via the PSI is more streamlined, easy and less time consuming in comparison to the other countries.
A gateway to work opportunities across the EU (post-registration)
It is a global hub of the pharmaceutical companies. It is the home country of about 85 pharma companies with 19 out of top 20 companies operating here.
Approximately 50 FDA-approved manufacturing plants are present in Ireland proving its dedication and commitment to international quality standards in pharmaceutical production.
Step-by-Step Pathway for Indian Pharmacists
1. Eligibility Check
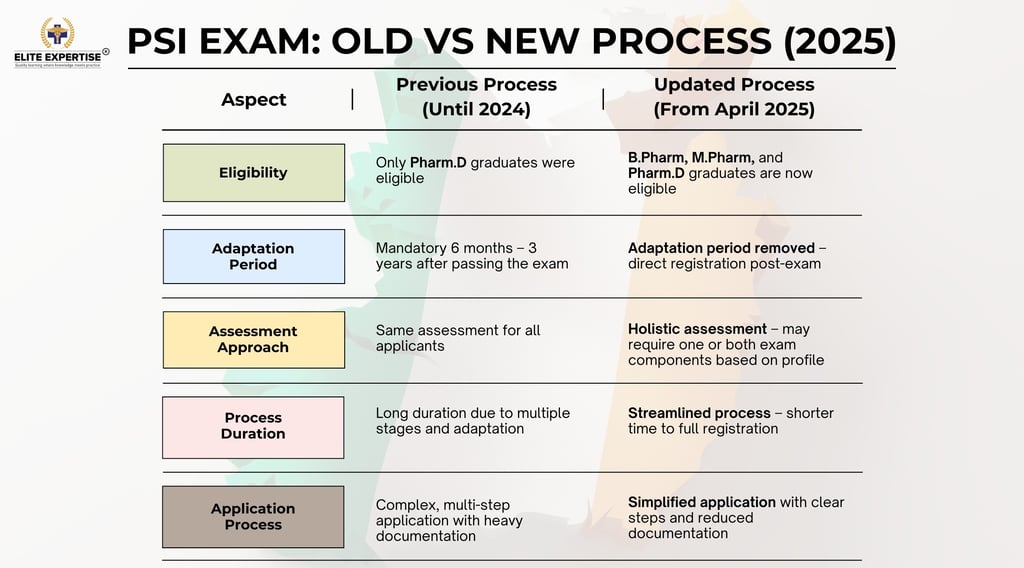
Indian pharmacy graduates with a Bachelor of Pharmacy (BPharm) or MPharm or PharmD degree from a recognised institution are eligible
A valid pharmacist registration in India (e.g., from PCI or State Pharmacy Council)
English language proficiency (IELTS Academic, TOEFL iBT, CAE or OET Pharmacy accepted)--could add this as TOEFL iBT and CAE are also accepted
2. Apply for Qualification Recognition: Stage 1
As a first step you need to submit an application to the PSI for recognition of your qualifications. This is a mandatory step for not only Indian pharmacists but all the countries fall under the TCQR pathway or Third Country Qualification Recognition Pathway.
For those do don’t know about the TCQR pathway, it's the official process managed by the Pharmaceutical Society of Ireland (PSI) for pharmacists who obtained their pharmacy degree and training in a country outside of the European Union (EU) or European Economic Area (EEA).
Documents required:
Academic transcripts
Degree certificates
Proof of registration as a pharmacist in India
Passport copy
Statement of professional experience (if any)
You will submit these through the PSI Recognition of Non-EU Qualification process.
To Apply for qualification process please download the relevant application forms from the following links:
TCQR2 Statutory Declaration Form
3. Holistic Assessment:
What happens after I submit my application?
Stage 2: Holistic assessment where if an applicant possesses a qualification appropriate for practice, independent external assessors will carry out holistic paper-based assessments of the application and supporting documentation.
The assessment will consider three components:
Input component: assess the qualification of the applicant and training that they have gone through during education.
Quality component: The quality assurance components of the regulatory system in place in the country where the applicant’s qualification was obtained, for example, whether the applicant’s primary qualification was accredited
Output component: Assesses th applicant’s relevant post-graduate education, work experience and continuing professional development.
The input and quality component are assessed by external assessor.
If they are considered equivalent to Irish standards next step is assessing the Output component.
After reviewing your documents, PSI may:
Grant automatic recognition (rare for non-EU degrees)- PATH A
Direct you to take an equivalency exam- PATH B
Refer you to the Third Country Qualification Recognition (TCQR) process, which includes a written exam and practical assessment
Indian pharmacists are usually required to complete the TCQR process.
4. PATH B:
If there are any deficiency in your application you can go through examination stage
If required, you will be asked to appear for:
Multiple-Choice Questionnaire (MCQ) test: Computer-based test covering pharmaceutical knowledge, clinical practice, and Irish pharmacy law.
Objective Structural Clinical Examination (OSCE): May include practical style clinical stations assessing the simulated real life case studies.
5. Certification of registration:
After successfully completing all exam you can apply for full registration with the PSI.
Once registered, you can work as a pharmacist in Ireland and even explore mobility across the European Union under mutual recognition agreements.
English Language Requirements
Majority of the Indian pharmacists find difficulty with the language as English is not the native language. Therefore, one must prove the English language proficiency through tests accepted by PSI as communication is the key for this job role.
Indian pharmacists must demonstrate proficiency in English via:
IELTS Academic: Overall score ≥ 7.0, no band < 6.5
OET Pharmacy: Minimum B grade in all sub-tests
Job Prospects in Ireland for Pharmacists
Once registered, you can apply for roles such as:
Community Pharmacist
Hospital Pharmacist
Clinical Pharmacy Specialist
Regulatory Affairs roles
Academic and Research positions
Tips for Indian Pharmacists
Keep all your academic and registration documents in order
Start preparing early for PSI’s written and clinical assessments
Improve communication skills for patient-facing roles
Consider guidance from professional coaching or mentoring platforms like Elite Expertise for exam and adaptation support
How Elite Expertise Can Help
At Elite Expertise, we support Indian pharmacists with:
PSI exam guidance
Comprehensive material for preparation
Guidance with document evaluation
Career counselling for Ireland and other countries (Australia, Canada, New Zealand) just to support the students. Avail this service talk to our 24*7 available admin team to get best options for you
One to one doubt consultation sessions
Mock tests curated for PSI exams
Conclusion
Working abroad is every man’s dream in India. The respect for the pharmacists attracts the Indian even more. The process is tough but you could achieve it with right guidance and preparation. Indian pharmacists can successfully register and build a rewarding career in Ireland via the TCQR process. With high demand for skilled professionals, Ireland remains one of the most promising destinations for internationally trained pharmacists.
⚠️ Disclaimer
This blog is for informational purposes only and reflects our views and interpretations. While we strive for accuracy, licensing requirements and regulations may change. Readers are advised to verify information with official sources such as the Pharmaceutical Society of Ireland (PSI) and the Health Products Regulatory Authority (HPRA) before making any decisions. We do not assume responsibility for any actions taken based on the information provided.


About the Author
Sahana Rao
Sahana Chinthapatla is renowned for her expertise in scientific and medical writing, backed by an MPharm in Pharmacology and years of experience in research analysis and scientific writing.
As the Head Business Writer at Elite Expertise, she provides insightful and well-researched content on KAPS, OPRA, PEBC, PSI and global pharmacy pathways, guiding aspiring pharmacists in their professional journeys.
Follow On
Head Business Writer | Elite Expertise
Follow Us
+91 76750 84909
Privacy Policy | © 2025 Elite Expertise . All Rights Reserved.
ELITE EXPERTISE PTY. LTD (ABN: 15668292439) (ACN: 668292439)
Australian Statutory Education License: OPP 2025 ELITE EXPERTISE PTY. LTD
Disclaimer
Elite Expertise is an online education platform dedicated solely to providing coaching and preparation services for the OPRA, PEBC, PSI and PTE exams. We do not offer any sponsorship or migration services. All information provided on our platform is for educational purposes only and should not be interpreted as legal or immigration advice. For inquiries regarding sponsorship, visa applications, or migration services, please consult with licensed immigration professionals or relevant authorities.
Elite Expertise is a trusted and results-driven training platform specializing in preparation for international pharmacist licensing exams. Our comprehensive courses, expert instructors, and proven methodologies have helped countless pharmacy professionals achieve their goals and succeed in competitive regulatory exams. We are proud of our strong success rate and commitment to excellence.
Elite Expertise is an independent training provider. We are not affiliated with any global pharmacy regulatory authorities or official exam-conducting bodies.
Copyright © 2026 Elite Expertise. All rights reserved.
Address
Unit 1/73 Beverley St, Doncaster East VIC 3109, Australia
